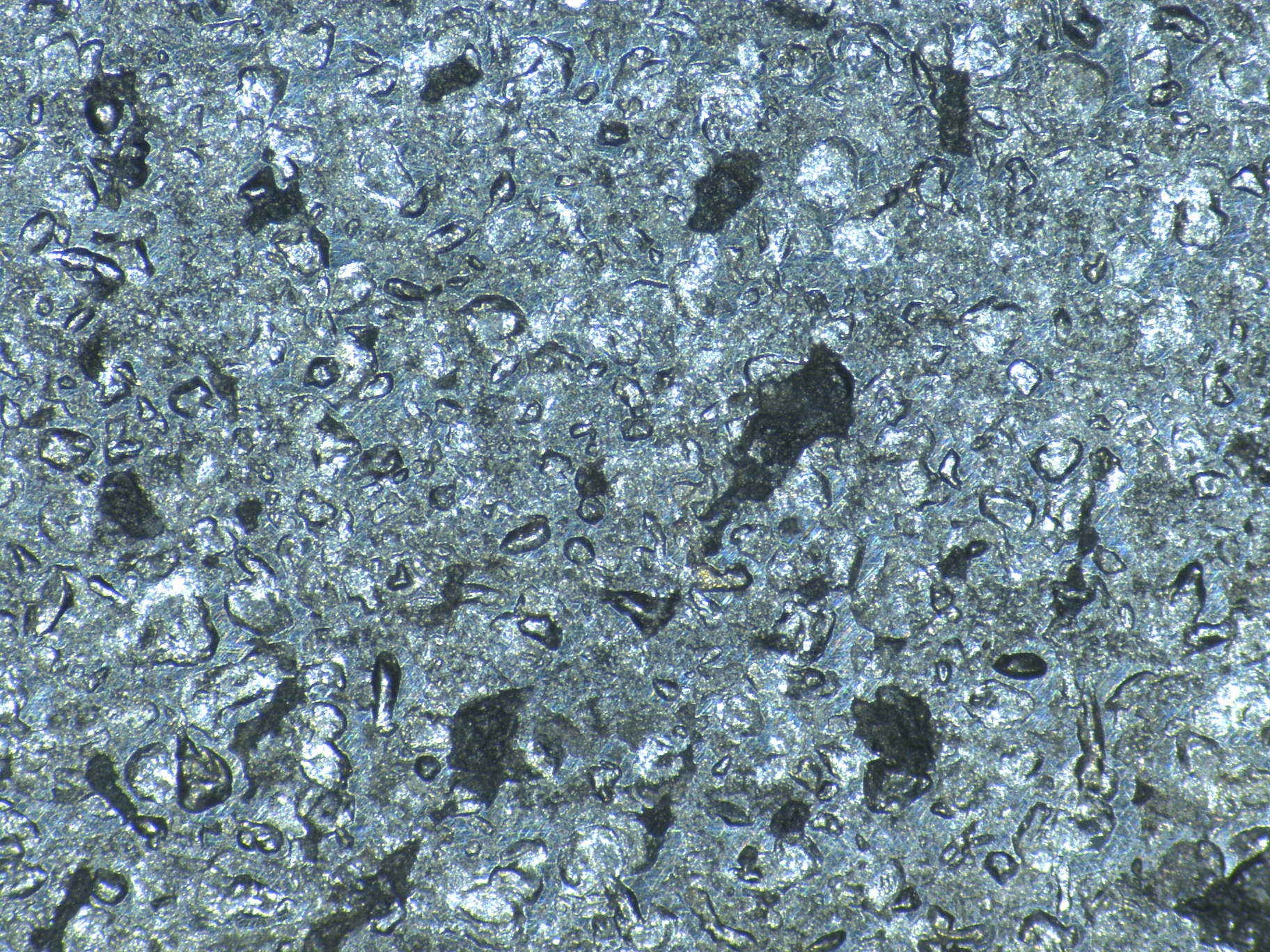
PITTING
Stainless steels, nickel-base and chromium-bearing alloys may be subjected to pitting and critical pitting temperature investigation. Test pieces are immersed in a ferric chloride solution (FeCl3) at constant temperature for a specified time, then are rinsed, weighed and examined. Following which, specimen mass loss is calculated and the number of pits (in order to establish the pit density) together with the maximum and average pit depth are recorded.
The ASTM G 48 standard test method provide the procedures to detect the critical temperature at which pitting corrosion initiates. The starting temperature is estimated by an equation multiplying chrome, nickel and molybdenum percentages and standard coefficients. If 0,025 mm or deeper pits start at this temperature, the test is repeated at a lower temperature; otherwise the test is repeated at a higher temperature. This method allows to detect the actual critical pitting temperature of the material.
TEST METHODS
- ASTM A1084 Method C
- ASTM G48 Method A, Method C, Method E
- ASTM G66
- EN ISO 9626
- ASTM A923 Metodo C
PHENOMENON DESCRIPTION
The term “pitting ” refers to a localized form of corrosion by which cavities or “holes” are produced in the material. It is initiated by local breakdown of the protective oxide film covering metals.
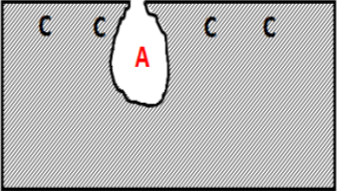
The cavity of the pit acts as an anode, ions migrate there, pH decreases and oxygen is consumed. The surrounding area, on the contrary, acts as a cathod and this causes an electrochemical process. The corrosion rate increases proportionally with the ratio of cathodic to anodic areas.
MORE INFORMATION
Ask a question or request a quote fulfilling the mask below
or call us at (+39) 0523 881 900
Required fields marked with *
YOU NEED ANOTHER TEST?
SIDERTEST
is ACCREDITED FOR A WIDE RANGE OF TESTS